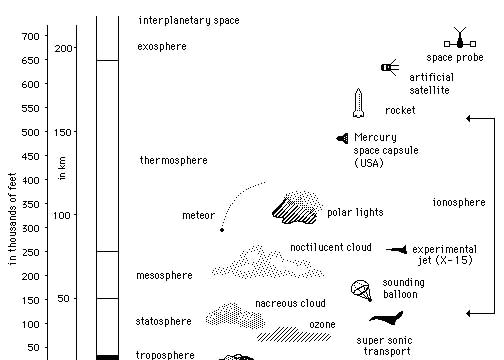

The earth's atmosphere is composed of several layers, distinguished by their primary constituents and by temperature variations from one layer to the next. Its main ingredients are nitrogen (78%) and oxygen (21%). The remaining 1% is comprised of water vapor and carbon dioxide, with additional trace elements including ozone, argon, neon, helium, hydrogen, methane, and xenon. 80% of the mass of the atmosphere is found in the very thin layer closest to the surface known as the troposphere. It is characterized by temperatures that decrease with increasing altitude. This is where all weather occurs. The ozone layer is found high above the troposphere in the stratosphere, where it absorbs most of the sun's harmful ultraviolet radiation. UV rays split oxygen molecules (O2) into atoms, which recombine with other O2 molecules to form ozone (O3). Students should distinguish between the protective ozone layer and the harmful ozone pollution found in the troposphere, near ground level.
Since the arrival of the industrial age, people have pumped billions of tons of pollutants into the air, causing many health problems and damaging the environment. The burning of fossil fuels at power plants, and in motor vehicles, has caused much of the problem. 60 million metric tons of carbon monoxide are added to the atmosphere each year in the US. In urban areas, smog (man-made ozone) severely decreases air quality for people, and reduces growth rates for trees and crops. Smog or ozone is formed when sunlight acts on a mixture of nitrogen oxides and volatile organic compounds. Nitrogen oxides and volatile organic compounds (hydrocarbons) are formed from the incomplete combustion of fossil fuels. Cities are most affected by smog during summer months because the sun plays an important role in its formation. Photochemical reactions of sunlight and gasoline fumes produce harmful ozone as well as deadly carbon monoxide(CO). CO is readily picked up by the hemoglobin in our red blood cells, and transported to the other cells in the body. CO and carbon dioxide (CO2) also contribute to the greenhouse effect, a natural process by which the atmosphere traps heat close to the earth's surface. Click here to read more about greenhouse gases. The rapid increase of these gases over the past century has raised the alarm concerning global warming, with its scenarios of agricultural impacts, rising sea levels and flooded coastal communities.
While one type of pollution may cause long term warming, another type is raising the specter of short term global cooling. Tiny bits of ash, metal, dust, and other solids called aerosols (suspended solid or liquid particles) are put into the atmosphere from motor vehicles, wood-burning stoves and fireplaces, volcanic ash, forest fires, and burning of forests or agricultural byproducts. It is believed that these particles might increase the reflectivity of the atmosphere, decreasing the total amount of solar radiation reaching the earth's surface. Click here to read more about Volcanoes and Global Climate Change. Particulates can also cause respiratory ailments, lung disease, and cancer. Nitrogen oxides and sulfur dioxide come from the burning of coal and other fossil fuels. These chemicals cause lung damage, and react with moisture in the atmosphere to produce acid rain, which damages forests, buildings, and statues. In recent years, evidence is mounting that the protective ozone layer may be affected by increasing levels of chloro-fluorocarbons (CFCs). CFCs are released into the atmosphere from refrigeration systems, jet fuels, aerosol sprays, Styrofoam, solvents, and air conditioners. The thinning of the ozone layer could result in higher levels of UV radiation reaching the earth's surface, causing a rise in skin cancer, immune system breakdown, and eye complications. Animals and plants alike are at risk if UV levels increase.